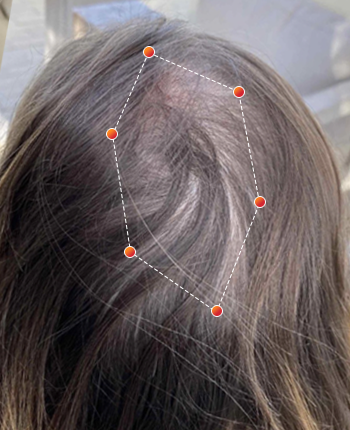
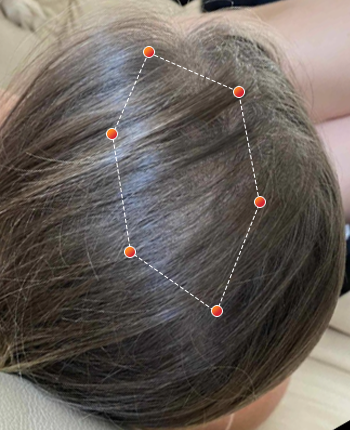
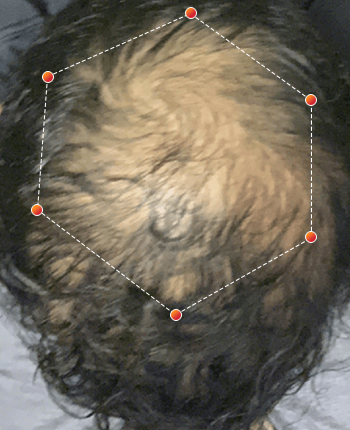
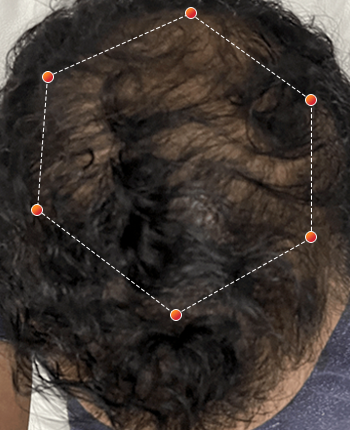
Clinical Results
The REVIAN 101 Clinical Trail was conducted from April 2017 to May 2019. The results were clear.
REVIAN Red is clinically proven to grow more hair in less time.
Clinical results
In 2017, we conducted a double-blind, placebo-controlled study, enrolling men and women ages 18-65 with pattern baldness (androgenetic alopecia), the study results demonstrate that REVIAN is an effective alternative to chemical-based topicals and prescription drugs.
Significant Results
In the medical community, what do health-care providers use to measure success?
Hairs grown per centimeter squared is the metric relied upon most and is used in the majority of well controlled hair loss clinical trials.
Rogaine® is a registered trademark of Johnson & Johnson, Inc.
Propecia® is a registered trademark of MERCK & CO., Inc.
View references
New change in hair count

Red laser device
Minoxidil Rogaine®
Finasteride Propecia®
Clinically Proven to Grow More Hair
Participants who were treated with the red light therapy and were at least 80% compliant for the duration of the study had an average of 26.3 more hairs per cm² compared to those who wore a similar cap but did not have light therapy after 16 weeks.
Subjects treated with placebo continued to lose hair over the duration of the study given the severity of pattern hair loss enrolled into the study (men with Norwood-Hamilton classifications of IIa – V and women with Ludwig-Savin Scale I-1 to I-4, II -1, II-2 or frontal patterns of hair loss).
View references

How does REVIAN compare?
Hair loss can be reversible with the right treatment. But to do so, you need to address the underlying problem. REVIAN’s dual wavelength technology releases nitric oxide and targets the three main pathogenic factors known to be associated with androgenetic alopecia: reduced blood flow, inflammation, and elevated levels of dihydrotestosterone (DHT).
 |
Low-Level Laser Therapy (LLLT) |
Minoxidil (Rogaine®) | Finasteride (Propecia®) | Follicular Transplant |
|
|---|---|---|---|---|---|
| Promote blood flow | |||||
| Reduce inflammation | |||||
| Inhibit DHT |
| Promote blood flow |
Reduce inflammation |
Inhibit DHT |
|
|---|---|---|---|
 |
|||
| Minoxidil (Rogaine®) | |||
| Finasteride (Propecia®) | |||
| Follicular Transplant |
Rogaine® is a registered trademark of Johnson & Johnson, Inc.
Propecia® is a registered trademark of MERCK & CO., Inc.
Grow more hair, quicker
REVIAN is clinically proven to grow more hair in less time than other hair loss products.
Finasteride
After 16 weeks, subjects treated with REVIAN had, on average, >80% more total hairs than those treated with once daily finasteride for 26 weeks.*
Drawbacks
Reoccurring monthly cost (forever). Systemic therapy that requires a doctor visit. Potential side effects include lower sex drive and erectile dysfunction.
Minoxidil
After 16 weeks, subjects treated with REVIAN had, on average, 30% more total hairs than those treated with twice daily 5% minoxidil topical foam.*
Drawbacks
Reoccurring monthly cost (forever). Must be used 2x daily. Goopy, messy chemical treatment with potential side effects including skin rash and irritation.Low-level laser therapy
Compared to a red laser device, REVIAN provides a more uniform scalp coverage & precise penetration with greater hair counts in significantly less time.*
Drawbacks
Lasers provide uneven light distribution. Most are bulky, corded or handheld devices that limit ease of use and mobility.
*No head-to-head trials were conducted. Percentages derived from cross study comparisons to published results for net change in hair counts vs. placebo/control arm.
Compliance made simple
For any hair growth regimen to work, compliance is key. Failure to use the product is almost certain to lead to a failure in new hair growth. The REVIAN RED app tracks your daily progress while providing a direct link to our team so that we are with you every step of the way.
In fact, participants in the REVIAN clinical trial, that had at least 80% compliance to the once daily, 10-minute treatment regimen grew more hair on average than the broad study population.

Trial references
- REV-01 Data on File. A multicenter, double-blind, placebo controlled study was conducted enrolling both men and women ages 18 and older with androgenetic alopecia. Participants were randomized to wear either the REVIAN RED cap or a Placebo Cap (no red light) for 10 minutes per day. Approximately half of the subjects had treatment with REVIAN RED light therapy; and half did not. Total hair counts were obtained from computer assisted scans of digital photographs taken of a defined target area (1 cm 2) centered around a tattoo located in the anterior mid area of the scalp. ClinicalTrials.gov Identifier: NCT04019795.
- Jimenez et al. Efficacy and Safety of a Low-level Laser Device in the Treatment of Male and Female Pattern Hair Loss: A Multicenter, Randomized, Sham Device-controlled, Double-blind Study. American Journal of Clinical Dermatology 2014. PubMed Reference.
- Olsen et al. A multicenter, randomized, placebo-controlled, double-blind clinical trial of a novel formulation of 5% minoxidil topical foam versusplacebo in the treatment of androgenetic alopecia in men. Journal of the American Academy of Dermatology 2007. PubMed Reference.
- Leyden et al. Finasteride in the treatment of men with frontal male pattern hair loss. Journal of the American Academy of Dermatology 1999. PubMed Reference.
Trial references
Primary Endpoint: Target Area Hair Counts at Week 16
Mean results shown for the Efficacy Evaluable Population of trial participants who completed all 16 weeks, had no major protocol violations, and who were at least 80% compliant with the treatment regimen for the duration of the trial. For full trial information, see ClinicalTrials.gov : NCT04019795